E-Z-GO TXT - Electric Owner Manual - Page 36
Hydrometer, Using A Hydrometer - golf cars manual
 |
View all E-Z-GO TXT - Electric manuals
Add to My Manuals
Save this manual to your list of manuals |
Page 36 highlights
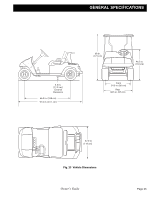
OPERATION AND SERVICE INFORMATION Read all of manual to become familiar with this vehicle. Pay attention to all NOTICES, CAUTIONS, WARNINGS and DANGERS. BA cost effective way to identify a poorly performing battery is to use a hydrometer to identify a battery in a set with a lower than normal specific gravity. Once the particular cell or cells that are the problem are identified, the suspect battery can be removed and replaced. At this point there is nothing that can be done to salvage the battery; however, the individual battery should be replaced with a good battery of the same brand, type and approximate age. Specific gravity is the measurement of a liquid that is compared to a baseline. The baseline is water which is assigned a base number of 1.000. The concentration of sulfuric acid to water in a new golf car battery is 1.280 which means that the electrolyte weighs 1.280 times the weight of the same volume of water. A fully charged battery will test at 1.275 - 1.280 while a discharged battery will read in the 1.140 range. NOTICE Do not perform a hydrometer test on a battery that has just been watered. The battery must go through at least one charge and discharge cycle in order to permit the water to adequately mix with the electrolyte. The temperature of the electrolyte is important since the hydrometer reading must be corrected to 80° F (27° C). High quality hydrometers are equipped with an internal thermometer that will measure the temperature of the electrolyte and will include a conversion scale to correct the float reading. It is important to recognize that the electrolyte temperature is significantly different from the ambient temperature if the vehicle has been operated. Fig. 21 Hydrometer Hydrometer A hydrometer is used to test the state of charge of a battery cell. This is performed by measuring the density of the electrolyte, which is accomplished by measuring the specific gravity of the electrolyte. The greater the concentration of sulfuric acid, the more dense the electrolyte becomes. The higher the density, the higher the state of charge. To prevent battery explosion that could result in severe personal injury or death, never insert a metal thermometer into a battery. Use a hydrometer with a built in thermometer that is designed for testing batteries. Using A Hydrometer 1. Draw electrolyte into the hydrometer several times to permit the thermometer to adjust to the electrolyte temperature and note the reading. Examine the color of the electrolyte. A brown or gray coloration indicates a problem with the battery and is a sign that the battery is nearing the end of its life. 2. Draw the minimum quantity of electrolyte into the hydrometer to permit the float to float freely without contacting the top or bottom of the cylinder. 3. Hold the hydrometer in a vertical position at eye level and note the reading where the electrolyte meets the scale on the float. 4. Add or subtract four points (.004) to the reading for every 10° F (6° C) the electrolyte temperature is above or below 80° F (27° C). Adjust the reading to conform with the electrolyte temperature, e.g., if the reading indicates a specific gravity of 1.250 and the electrolyte temperature is 90° F (32° C), add four points (.004) to the 1.250 which gives a corrected reading of 1.254. Similarly if the temperature was 70° F (21° C), subtract four points (.004) from the 1.250 to give a corrected reading of 1.246. 5. Test each cell and note the readings (corrected to 80° F or 27° C). A variation of fifty points between any two cell readings (example 1.250 - 1.200) indicates a Page 20 Owner's Guide