Kyocera TASKalfa 3051ci Printing System (11),(12),(13),(14) Color Reference G - Page 61
The physics of color, spectral light
 |
View all Kyocera TASKalfa 3051ci manuals
Add to My Manuals
Save this manual to your list of manuals |
Page 61 highlights
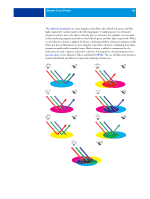
DESKTOP COLOR PRIMER 61 The physics of color The human eye can see electromagnetic radiation at wavelengths between 400 nanometers (purplish blue) and 700 nanometers (red). This range is called the visible spectrum of light. We see pure spectral light as intensely saturated or pure colors. Sunlight at midday, which we perceive as white or neutral light, is composed of light from across the visible spectrum in more or less equal proportions. Shining sunlight through a prism separates it into its spectral components, resulting in the familiar rainbow of colors illustrated in the following figure. Like the sun, most light sources we encounter in our daily environment emit a mixture of light wavelengths, although the particular distribution of wavelengths can vary considerably. Light from a tungsten light bulb, for example, contains much less blue light than sunlight. Tungsten light appears white to the human eye, which, up to a point, can adjust to the different light sources. However, color objects appear different under tungsten light than they do in sunlight because of the different spectral makeup of the two light sources. The mixture of light wavelengths emitted by a light source is reflected selectively by different objects. Different mixtures of reflected light appear as different colors. Some of these mixtures appear as relatively saturated colors, but most appear as grays or impure hues of a color.