Texas Instruments TI-36X Pro User Manual - Page 65
Coulombs per mole
 |
View all Texas Instruments TI-36X Pro manuals
Add to My Manuals
Save this manual to your list of manuals |
Page 65 highlights
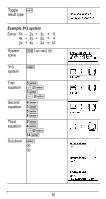
Note: Displayed constant values are rounded. The values used for calculations are given in the following table. Constant Value used for calculations c speed of light 299792458 meters per second g gravitational acceleration 9.80665 meters per second2 h Planck's constant 6.62606896×10M34 Joule seconds NA Avogadro's number 6.02214179×1023 molecules per mole R ideal gas constant 8.314472 Joules per mole per Kelvin me electron mass 9.109381215×10M31 kilograms mp proton mass 1.672621637×10M27 kilograms mn neutron mass 1.674927211×10M27 kilograms mμ muon mass 1.88353130×10M28 kilograms G universal gravitation 6.67428×10M11 meters3 per kilogram per seconds2 F Faraday constant 96485.3399 Coulombs per mole a0 Bohr radius 5.2917720859×10M11 meters re classical electron 2.8179402894×10M15 meters radius k Boltzmann constant 1.3806504×10M23 Joules per Kelvin e electron charge 1.602176487×10M19 Coulombs u atomic mass unit 1.660538782×10M27 kilograms atm standard 101325 Pascals atmosphere H0 permittivity of vacuum 8.854187817620×10M12 Farads per meter m0 permeability of vacuum 1.256637061436×10M6 Newtons per ampere2 Cc Coulomb's constant 8.987551787368×109 meters per Farad 65